Here you are: IMGT Web resources > IMGT Lexique
Polarity
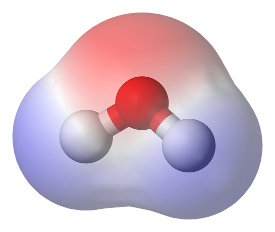
Even though the total charge on a molecule is zero, the nature of chemical bonds is such that there may some separation of charge in the chemical bonds, so that one part of the molecule has a positive charge and the other a negative charge. Such molecules have a dipole moment and are said to be 'polar'.
A good example is the water molecule (H2O). The electrons of hydrogen atoms are strongly attracted to the oxygen atom; thus, the oxygen has a negative charge (red shade) while the hydrogens have a positive charge (blue shade), giving the water molecule an effective dipole moment.