Polypeptide chain
Steric limitations on the bond angles in a polypeptide chain.
Peptide bonds
Each amino acid contributes three bonds to its polypeptide chain
- the peptide bond is planar and does not permit rotation.
- rotation can occur about the N-Cα bond, whose angle of rotation is called phi (φ) and about the Cα -C bond, whose angle of rotation is called psi (Ψ).
R = amino acid side chain.
The conformation of the main-main atoms in a protein is determined by one pair of phi et psi angles for each amino acid; because of steric collisions within each amino acid, most pairs of phi and psi angles do not occur. In the Ramachandran plot, each dot represents an observed pair of angles in a protein (Richardson J. Adv. Prot. Chem. 34, 174-175 (1981)).
Hydrogen bonds
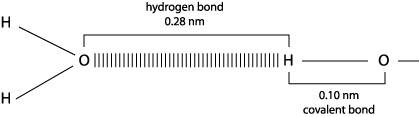
A hydrogen bond is formed when a hydrogen atome is shared between two other atoms, both electronegative, such as O and N.
Any molecules that can form hydrogen bonds to each other can alternatively form hydrogen bonds to water molecules. Because of this competition with water molecules, the hydrogen bonds formed between two molecules dissolved in water are relatively weak.
- Hydrogen bonds are strongest when the three atoms lie in a straight line.
- Hydrogen bonds have only about 1/20 the strength of a covalent bond.
Van der Waals forces
Each type of atom has a radius, known as its van der Waals radius, at which van der Waals forces are in equilibrium. Two atoms will be attracted to each other by van der Waals forces until the distance between them equals the sum of their van der Waals radii.
Although individually very weak, these van der Waals attractions can become important when two chains fit very close together. However, two atoms will very strongly repel each other if they are brougth too close together. This van der Waals repulsion plays a major role in limiting the possible conformations of a molecule.



